Heat Capacity Worksheet - Answer Key
Back to the other Thermodynamics Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- What is molar heat capacity?
The energy required to raise the temperature of 1 mol of a substance by 1K. Represented by, C.
- What is specific heat capacity?
The energy required to raise the temperature of 1 gram of a substance by 1K. Represented by, C.
- The kinetic energy of a system is directly related to its temperature. So if we heat a system we increase its kinetic energy.
- What are two conditions under which a gas can be heated?
- Constant Volume.
- Constant Pressure.
- What are the values for:
- Cv = 3/2 R
- Cp = Cv + R = 5/2 R
- Why is Cv<Cp?
Cp > Cv because energy gets used for heating and work in constant pressure situation. - When energy is added to a molecule there are three potential forms of motion that it can be transferred into:
- Translational – The molecule’s movement around the container
- Vibrational – The spring-like movement of the bond between atoms.
- Rotational – The spinning of the molecule on an axis.
- Translational motion is the only motion that directly affects temperature.
- The values 3/2 R and 5/2 R are only applicable to monatomic ideal gases. Why?
A monatomic ideal gas is only capable of undergoing translational motion.
- This means that the calculated molar heat capacities are often less than actual molar heat capacities.
- What equation relates ΔE to heat capacity?
ΔE = nCVΔT
- Under what conditions can you use this equation?
Under any all conditions.
- Under what conditions does ΔE = q?
When the volume is constant.
- What equation relates ΔH to heat capacity?
ΔH = nCpΔT
- Under what conditions can you use this equation?
Under any all conditions.
- Under what conditions does ΔH=q?
When the pressure is constant.
- Which value of R do we use in these equations?
R = 8.3145 J mol-1K-1
- Fill in the chart.
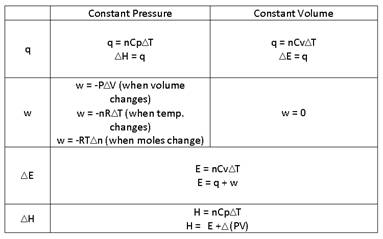
- Consider a sample containing 5 moles of a monatomic ideal gas that is taken from State Aà State B by 2 different paths. For each step, assume that the external P is constant and equals the final P of the gas for that step. Calculate the values of q, w, ΔH and ΔE for each step along the 2 paths and the totals for the 2 paths. What do the totals demonstrate?
Path A: q = 2.3 kJ Path B: q = 14.4 kJ
w = 9.1 kJ w = – 3.04 kJ
ΔE = 11.4 kJ ΔE = 11.4 kJ
ΔH = 19.0 kJ ΔH = 19.0 kJ
The values of q and w are path dependent.
The values of ∆H and ∆E are path independent.
