Vapor Pressure Worksheet - Answer Key
Back to the other Solution Chemistry Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- What is Vapor Pressure?
This is the pressure above a solid or liquid due to evaporation.
- Can a solute affect the vapor pressure of a solvent?
Yes.
- What do solutes do to the vapor pressure of the solvent?
Lower it.
- How does it lower vapor pressure of solvent?
Less of the solvent is able to escape due to its path for evaporation being blocked by the new solute particles; less particles escaping means lowered vapor pressure.
- What are two categories of solute?
- Non-volatile Solute
- What is Raoult’s Law for this type of solute?
Psol’n = χA solvent (Posolvent)
- Volatile Solute
- What is Raoult’s Law for this type of solute?
Psol’n = PA + PB = χA solvent ( Posolvent) + χsolute (Posolute)
- What type of solution obeys Raoult’s Law?
Ideal Solutions.
- How does an ideal solution differ from a non-ideal solution?
In an ideal solution there is no interaction between molecules.
- How do these interactions affect the predictions of Raoult’s Law?
If there are strong interactions, the IMFs are higher than for the pure substances.
If there are weak interactions, the IMFs are lower than for the pure substances.
- Label which of the following graphs obeys Raoult’s Law, which has strong exothermic interactions and which has endothermic interactions that cause a deviation from Raoult’s Law.
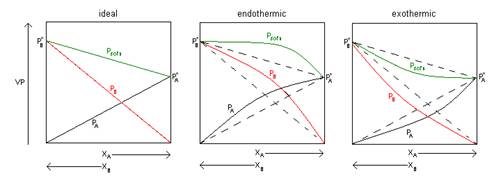
- Give an example of each type of interaction
- Benzene and Toluene = Ideal
- CH3CH2OH + C6H14 = endothermic
(polar and nonpolar combination)
- C3H6O + H2O = exothermic
(polar and polar combination)
- When pure methanol is mixed with water, the solution gets warmer to the touch. Would you expect this solution to be ideal? Why or why not?
No. We would expect this solution to have a negative deviation from an ideal sol’n as it was an exothermic reaction.
- Given

Which of the following statements is false concerning solutions of A and B?
- The solutions exhibit negative deviations from Raoult’s Law.
- ∆Hmix for the solutions should be exothermic.
- The intermolecular forces are stronger in solution than in either pure A or B.
- Pure liquid B is more volatile than pure liquid A.
- The solution with χB = 0.6 will have a lower boiling point than either pure A or pure B.
- Which of the following will have the lowest total vapor pressure at 25oC? Which has the highest vapor pressure at 25oC? At 25oC, the vapor pressure of pure water is 23.8 torr.
- Pure water.
- A solution of glucose in water with χglucose = 0.01
- A solution of sodium chloride in water with χNaCl = 0.01
- A solution of methanol in water with χmethanol = 0.2
(at 25oC, the vapor pressure of pure methanol is 143 torr)
The order in vapor pressure for these solutions would be:
d > a > b > c
- Glycerin (C3H8O3) is a nonvolatile liquid. What is the vapor pressure of a solution made by adding 164 g of glycerin to 338 mL of H2O at 39.8°C? The vapor pressure of pure water at 39.8°C is 54.74 torr and its density is 0.992 g/cm3.
Psol’n = 50.0 torr
- At a certain temperature the vapor pressure of pure benzene (C6H6) is 0.930 atm. A solution was prepared by dissolving 10.0 g of a non-dissociating, nonvolatile solute in 78.11 g of benzene at that temperature. The vapor pressure of the solution was found to be 0.900 atm. Assuming that the solution behaves ideally, determine the molar mass of the solute.
Molar Mass = 302.5 g/mol
