Concentration Cells Worksheet
Back to the other Electrochemistry Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- Consider the following cell:
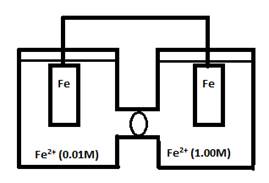
- What would ε° for this cell be?
- Will this cell run?
- What would the relative value of ε be?
- What is the driving force behind this cell?
- In what direction would this cell run?
- What equation would you use to calculate ε?
- What would ε° for this cell be?
- Consider the following cell when [Ni2+] in the left cell has the following concentrations. For each case, indicate the cathode, anode, and direction of e- flow.
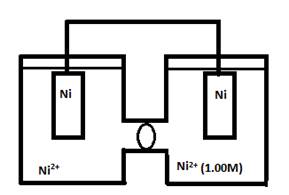
- 1.0M
- 2.0M
- 0.10M
