Concentration Cells Worksheet - Answer Key
Back to the other Electrochemistry Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- Consider the following cell:
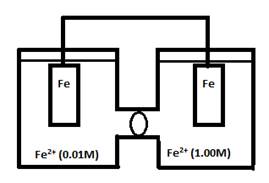
- What would ε° for this cell be?
εocell = 0
- Will this cell run?
Yes.
- What would the relative value of ε be?
The value of εocell would be greater than zero.
- What is the driving force behind this cell?
Entropy drives this reaction forward. It is more entropically favorable to have equal concentrations on both sides.
- In what direction would this cell run?
This cell runs from lower concentration to higher concentration.
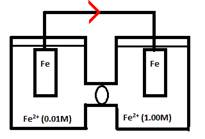
If electrons are being lost by the left compartment (the anode) the following reaction would be taking place:

From this you can see that [Fe2+] would increase in the left compartment.
The right compartment (the cathode) would be gaining electrons:
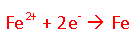
Thereby lowering the concentration of Fe2+ in the cathode.
This reaction will continue until the 2 compartments have an equal concentration of Fe2+.
- What equation would you use to calculate ε?
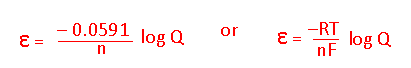
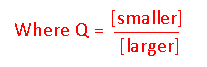
- True or False.
A concentration cell is a type of galvanic cell.
True. A concentration cell runs on a spontaneous reaction.
- Consider the following cell when [Ni2+] in the left cell has the following concentrations. For each case, indicate the cathode, anode, and direction of e- flow.
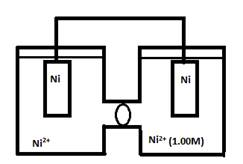
Remember that these concentration cells will run only if there is a difference in conentration between the 2 cells. Additionally, the electrons will flow from the less concentrated to more concentrated side.
- 1.0M
Concentrations are equal cell will not operate.
- 2.0M
The electrons will flow from right to left. The left compartment being the cathode and the right compartment being the anode.
- 0.10M
The electrons will flow from left to right. Thus making the right compartment the cathode and the left compartment the anode.
