Quantum Mechanics Practice Test
Back to the other Quantum Mechanics Practice Tests and other General Chemistry Practice Tests
Go To -> Practice Test - Answer Key
- Would light of wavelength (650 nm) be able to excite an electron in Be3+ from n=2 to n=6?
- Determine the number of orbitals that can have the following designation
- n = 4
- n = 3 ml = 2
- 2p
- How many electrons can have the following designation
- 3d ml = 0 ms = + ½
- n = 6 ml = 2
- Determine if the energy is absorbed or emitted when an electron transitions from the n=7 to the n=2 energy level of C5+ and the wavelength of the photon associated with the transition.
- It takes 208.4 kJ of energy to remove 1 mole of electrons from the atoms on the surface of rubidium metal. If rubidium metal is irradiated with 254-nm light, what is the maximum velocity of the released electron?
- Consider and atom traveling at 2.78 x 108 m/s. The De Broglie wavelength is found to be 1.797 x 10-5 pm. Which element is this?
- What is the electron configuration for
- Fe2+
- As 2-
- Given:
The figure below represents part of the emission spectrum for a one-electron ion in the gas phase. All the lines result from the electronic transition to the n = 3 state.
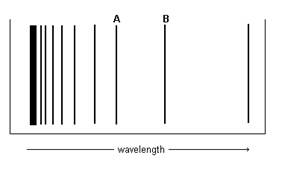
- What electronic transition corresponds to lines A and B?
- If the wavelength of line B is 142.5 nm, calculate the wavelength of line A.
