Oxidation Reduction Reactions Worksheet - Answer Key
Back to the other Reactions Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- What is an oxidation-reduction (or redox) reaction?
This is a reaction in which electrons are transferred between reactants.
- What do the following terms mean?
- Oxidation
Oxidation is a loss of electrons
- Reduction
Reduction is a gain of electrons
- What is a helpful way to remember this?
A mnemonic device to help you remember:
OIL RIG (Oxidation Is Loss Reduction Is Gain)
- What is an oxidation number?
An oxidation number allows one to keep track of electron flow in a reaction.
- Assign the oxidation number for the following
- KMnO4
Oxidation Numbers
K: +1
Mn: +7
O: –2 (for each oxygen)
- (NH4)2HPO4
Oxidation Numbers
N: –3
H: +1 (for each hydrogen)
P: +5
O: –2 (for each oxygen)
- Fe3O4
Oxidation Numbers
Fe: +8/3
O: –2 (for each oxygen)
- XeOF4
Oxidation Numbers
Xe: +6
O: –2
F: –1 (for each fluorine)
- What are the practical applications of oxidation numbers?
They allow us to determine what has been oxidized and what has been reduced.
- Consider:
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (g)
Determine what was oxidized and what was reduced.
C: -4 → +4 Carbon became less negative (lost electrons, oxidized)
H: +1 → +1 Hydrogen didn’t undergo change (unimportant)
O: 0 → -2 Oxygen gained negativity (gained electrons, reduced)
- What is an
- Oxidizing agent?
The oxidizing agent is the reactant that contains the species that gets reduced.
- Reducing agent?
The reducing agent is the reactant that contains the species that gets oxidized.
- Consider:
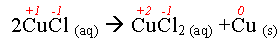
Cu: +1 → +2 Lost electrons → oxidized
Cl: -1 → -1 Nothing
Cu: +1 → 0 Gained electrons → reduced
determine the following:
- What was oxidized?
Copper
- What was reduced?
Copper
- What was the reducing agent?
CuCl
- What was the oxidizing agent?
CuCl
- Consider:
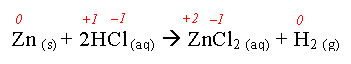
Zn: 0 → +2 Lost electrons → oxidized
H: +1 → 0 Gained electrons→ reduced
Cl: -1 → -1 Nothing
- What was oxidized?
Zinc
- What was reduced?
Hydrogen
- What was the reducing agent?
Zn
- What was the oxidizing agent?
HCl
- What are the steps to balancing a redox reaction using the ½ reaction method?
1. Break the reaction up into two half reactions. One for is the
reduction and the other is the reduction.
2. Balance all elements in the reaction except for oxygen and hydrogen.
3. Balance the oxygen by adding H2O.
4. Balance the hydrogen by adding H+.
5. Balance charge by adding electrons.
6. Equalize the number of electrons coming out of one reaction with
those going into the other.
7. Add up the two reactions. Make sure to cancel out where necessary.
. .
If you asked to balance under acidic conditions – you can stop after this step. If you are asked to balance under basic conditions, you will need to proceed onto step 8.
. .
8. Neutralize the H+ by adding in the same number of moles of –OH. Make sure to add the –OH to both sides of the reaction.
- Balance the following redox reaction using the ½ reaction method just described under both acidic and basic conditions.
MnO4 (aq) + H2C2O4 (aq) → Mn2+(aq) + CO2 (g)
Acidic Conditions:
6 H+ + 2MnO4- + 5 H2C2O4→ 2 Mn2+ + 8 H2O + 10 CO2
Basic Conditions:
2MnO4- + 5 H2C2O4→ 2 Mn2+ + 2 H2O + 10 CO2 + 6 -OH
- A 45.20 mL sample of sol’n containing Fe2+ ions is titrated with a 0.225M KMnO4 sol’n. It required 23.51 mL of KMnO4 sol’n to oxidize all the Fe2+ ions to Fe3+ by the following reaction
MnO4- (aq) + Fe2+(aq) → Mn2+(aq) + Fe3+(aq)
What was the concentration of Fe2+ in the sol’n?
0.0585 M Fe2+ - Consider:
Na2SO4 (aq) + Pb(NO3)2 (aq)→
- What are the three types of reaction equations that can be used to describe this reaction?
- What products, if any, form? How could you tell if no product was formed?
- Consider the reaction between silver nitrate and calcium chloride
- How many grams of silver chloride can be prepared by the reaction of 100.0 mL of 0.20M silver nitrate with 100.0 mL of 0.15 M calcium chloride?
- Calculate the concentration, in M, for each of the ions remaining in sol’n after reaction has gone to completion.
- How many grams of silver chloride can be prepared by the reaction of 100.0 mL of 0.20M silver nitrate with 100.0 mL of 0.15 M calcium chloride?
- What volume of 0.900M Na3PO4 is required to precipitate all the lead (II) ions from 160.0 mL of 0.650M Pb(NO3)2?
