Nuclear Chemistry Practice Test - Answer Key
Back to the other Nuclear Chemistry Practice Tests and other General Chemistry Practice Tests
Go To -> Practice Test - Answer Key
- A rock contains 0.466 mg of 206Pb for every 1.000 mg of 238U present. Assuming that no Pb was originally present, that all 206 formed over the years has remained in the rock, and that the number of nuclides in the intermediate stages of decay between 238U and 206Pb is negligible, calculate the age of the rock. (For 238U, t1/2=4.5 x 109 yr).
- 2.63 x 109 yr
- 2.79 x 109 yr
- 2.48 x 109 yr
- 1.87 x 109 yr
- 4.31 x 109 yr
- The stable nuclide
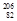 Pb is formed from
Pb is formed from 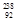 U by a long series of and decays. Which of the following nuclides could not be involved in this decay series?
U by a long series of and decays. Which of the following nuclides could not be involved in this decay series?
- Pa-234
- U-239
- Po-218
- Tl-210
- Rn-222
- Which of the following balanced equations is labeled incorrectly?
- Alpha emission:
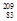 Bi +
Bi +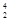 He à
He à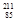 At + 2
At + 2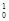 n
n - Fusion:
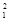 H +
H + 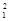 H à
H à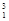 H +
H + 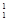 H
H - Bombardment:
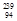 Pu +
Pu + 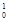 n à
n à 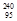 Am +
Am +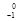 e
e - Beta production: beta production:
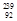 U à
U à 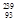 Np +
Np + 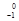 e
e - Alpha production:
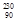 Th à
Th à 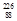 Ra +
Ra + 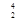 He
He
- Which statement is true about the following reaction?
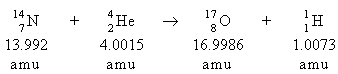
- Energy is absorbed in the reaction.
- Energy is released in the reaction.
- No energy change is associated with the reaction.
- Not enough information is given for us to determine the energy change.
- A positron and electron annihilate each other upon colliding, thereby producing energy:
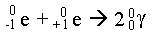
Assuming that both γ rays have the same energy, calculate the wavelength of the electromagnetic radiation produced.
2.42 x 10-12 m
- The binding energy per nucleon for magnesium – 27 is 1.326 x 10-12 J/nucleon. Calculate the atomic mass of magnesium – 27.
26.98 g/mol
- Naturally occurring uranium is composed mostly of 238U and 235U, with relative abundances of 99.28% and 0.72%, respectively. The half-life for the 238U is 4.5 x 109 years and the half=life for the 235U is 7.1 x 108years. Assuming that the earth was formed 4.5 billion years ago, calculate the relative abundances of the 238U and 235U isotopes when the earth was formed.
238U = 77%
235U = 23%
