Electrochemistry Practice Test - Answer Key
Back to the other Electrochemistry Practice Tests and other General Chemistry Practice Tests
Go To -> Practice Test - Answer Key
- Consider the following voltaic cell
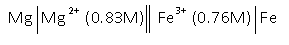
Determine the cell potential after the reaction has run long enough for the [Mg2+] to have changed by 0.36M.
E = 2.32 V
- The solubility product for CuI(s) is 1.1 x 10-12. Calculate the value of Eo for the half reaction at 298K:
CuI + e-→ Cu + I-
Eo = –0.19V
- Calculate the pH of the cathode compartment for the following reaction given. εcell = 2.99 V when [Cr3+] = 0.15 M, [Al3+] = 0.30 M, [Cr2O7 2 -] = 0.55 M
2Al(s) + Cr2O7 2-(aq) + 14H+(aq)→ 2Al3+(aq) + 2 Cr3+(aq) + 7H2O(l)
pH = 0.174
- What time is required to plate a metal tray (24.0 cm x 12.0 cm) with a coating (thickness = 0.00200 cm) of silver (density = 10.5 g/cm3) using a current of 7.65 A?
23.6 minutes
- In which of the following cases can Eocell be equal zero?
- In any cell at equilibrium
- In a concentration cell
- Eo can never equal zero
- Both a and b
- Indicate for each of the following statements applies to Eocell, Ecell, to both or neither.
- Decreases as the cell reaction progresses
Eocell
- Doubles when the coefficients of the equation are doubled.
Neither
- Changes with temperature
Both
- Can be calculated from K
Eocell
- Is a measure of how far the cell reaction is from equilibrium.
Ecell
- A current of 15.0A electroplated 50.0 g of hafnium metal from an aqueous solution in 2.00 h. What is the oxidation number of hafnium in the solution?
4
- Suppose that 2.69 g of a silver salt (AgX) is dissolved in 550 mL of water. With a current of 3.5 A, 395.0 s was need to plate out all of the silver. What is the formula of the salt?
AgBr
- If you were to construct a concentration cell in which one-half contains 1.0 M CrCl3 and the other half-cell 0.0010 M CrCl3, and both electrodes were chromium, at which electrode would reduction spontaneously happen? How will each of the following changes affect the cell potential?
- Adding 100 mL pure water to the anode compartment.
Increase cell potential.
- Adding 100 mL 1.0 M NaOH (aq) to the cathode compartment (Cr(OH)3 is insoluble).
Decrease cell potential.
- Increasing the mass of the chromium electrode in the anode compartment.
No change.
- In a neuron, the concentration of K+ ions inside the cell is about 20 to 30 times as great as that outside. What potential difference between the inside and the outside of the cell would you expect to measure if the difference is due only to the imbalance of potassium ions? (Assume 25oC)
0.09V - For :
F2 (g) + 2H+(aq) + 2e - → 2HF(aq) Eo = +3.03 V.
Calculate Ka for HF.
Ka = 1.97 x 10-3
- An aqueous solution of Na2SO4 was electrolyzed for 30.0 min; 25.0 mL of oxygen was collected at the anode over for water at 22oC and a total pressure of 722 torr. Determine the current that was used to produce the gas. (the vapor pressure of water at 22oC is 19.83 torr).
I = 0.204 C/s
