Coordination Compounds Test - Answer Key
Back to the other Coordination Compounds Practice Tests and other General Chemistry Practice Tests
Go To -> Practice Test - Answer Key
- Give the number of geometric isomers for the square planar compound [MABCD] where M represents a metal and A, B, C, and D represent ligands.
- 1
- 2
- 3
- 4
- There are no geometric isomers
- The spectrochemical series is (going from weak to strong field):
I - < Br - < Cl - < F - < OH - < H2O < NH3 < en < NO2 - < CN -
Consider (1) [Co(I)6] 3- and (2) [Co(CN)6] 3-
- (1) is paramagnetic, (2) is not.
- (2) is paramagnetic, (1) is not.
- Both are paramagnetic
- None of them are paramagnetic
- Consider the complex ions Co(NH3)63+, Co(CN)63- and CoF63-. The reflected colors were violet, orange and green. Match the complex ion to the color reflected.
[Co(CN)6]3- orange
[CoF6]3- green
[Co(NH3)6]3+ violet
- Which of the following metal ions should be colorless? Pick all that apply.
- Ti4+
- Cu+
- Cr3+
- Sc2+
- Zn2+
- What is the electron configuration for the transition metal ion in the compound:
(NH4)2[Fe(H2O)2Cl4]
Fe2+: [Ar] 3d6
- Name the following compounds:
- K2[Ag(H2O)3Br3]
potassium triaquatribromoargentate(I)
- [Mn(NH2CH2CH2NH2)3]SO4
tris(ethylenediamine)manganese(II) sulfate
- [Pt(NH3)4I2][PtI4]
(hint: Pt is commonly found in the +2 or +4 oxidation state)
tetraamminediiodoplatinum(IV) tetraiodoplatinate(II)
- Sodium nitroprusside, Na2[Fe(CN)5(NO)], is an octahedral complex given to heart transplant patients. It contains the nitrosonium ligand, NO+, which is one of the few known cationic ligands. Given this, determine the oxidation state of Fe in sodium nitroprusside.
- -1
- 0
- +1
- +2
- +3
- How many unpaired electrons are present in sodium nitroprusside?
- 0
- 1
- 2
- 4
- 5
- For the reaction:
[Fe (CN)5Br]3- + Br-→ [Fe(CN)4Br2]3- + CN-
What would the predicted ratio of cis to trans isomer of the product?
4:1
- What is the name of the nonpolar complex ion, [Cr(H2O)4I2]+? Include cis/trans geometry.
trans – tetraaquadiiodochromium (III) ion
- What is the name of the polar complex ion, [CuCl2F2]2-? Include cis/trans geometry.
cis – dichlorodifluorocuprate (II) ion
- Which of the following metal ions should exhibit the largest crystal field splitting?
- Fe3+
- Ir3+
- Mn3+
- Co3+
- How many distinct geometric isomers are there in the figure below?
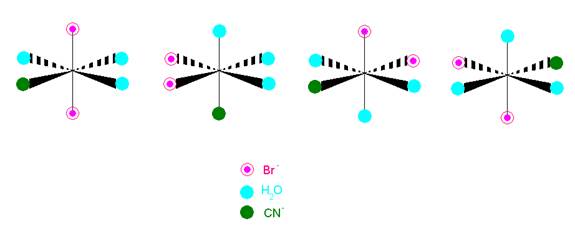
Three
- Is this compound capable of linkage isomerism? If so, through which ligand.
- This is compound only exhibits stereoisomerism.
- Yes, through the Br – ligand.
- Yes, through the H2O ligand.
- Yes, through the CN – ligand.
- b-d are correct.
- The complex CrL62+, where L is a neutral ligand, appears yellow. The number of unpaired d electrons in this complex ion is?
2 unpaired e-
- Give the number of geometric isomers for the octahedral compound [MA3B2C], where A, B and C represents ligands.
- 2
- 3
- 4
- 5
- 6
- Which metal has a d5 electron configuration?
- Pd2+
- Ag+
- Fe3+
- Os2+
- Co2+
- Excess Ag+ reacts with one mole of [COCl3(NH3)3]. How many moles of AgCl does this produce?
- 0
- 1
- 2
- 3
- 6
- Which of the following coordination compounds will form a precipitate when treated with an aqueous solution of AgNO3?
- [Cr(NH3)3Cl3]
- [Cr(NH3)6]Cl3
- Na3[Cr(CN)6]
- Na3[CrCl6]
- all of these
- Calculate the total number of unpaired electrons in the following complex ions:
Ni(H2O)62+, Fe(CN)42-, Co(NH3)62+.
7 unpaired electrons
- Give the number of geometric isomers for the octahedral compound [MAB4C], where A, B, and C represent ligands.
Two
- True or False
The complex [HgI4] 2- ion could be expected to appear green because I- is a weak field ligand.
