Matter vs. Energy Worksheet - Answer Key
Back to the other Quantum Mechanics Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key
- In the macroscopic viewpoint, energy has the characteristics of being
- Massless
- Delocalized
- Energy is described as a wave.
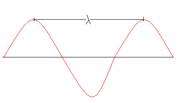
- What is the equation of a wave?
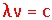
- What do each of the terms in the equation stand for?
λ – Wavelength (maximum to maximum or minimum to minimum, in meters)
ν – Frequency (# of waves passing through a point per second, in Hertz, s-1)
c – 2.998 x 108 m/s (speed of light).
- In the macroscopic viewpoint, matter has the characteristics of being
- Having mass.
- Localized.
- How are the following frequently classified?
- Ball - Matter
- Light – Energy
- Electron – Matter
- At the microscopic level is the distinction between matter and energy actually so black and white?
No.
- What are some experiments that initiated this change in perspective?
- Blackbody radiation – energy can only be absorbed in integer multiples.
- Double Slit Experiment – wave formation of electrons
- Photoelectric effect – particle properties of light.
