Electron Affinity Radius Worksheet - Answer Key
Back to the other Periodic Table Trends Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- What is electron affinity?
The energy change when an e- is added to a gaseous atom.
X(g) + e-→ X – (g)
An endothermic process.
- What element has the greatest electron affinity?
Chlorine has the greatest electron affinity. The reason that chlorine has a greater electron affinity than fluorine us that fluorine small size leads to some electron-electron repulsion – making it less favorable.
- General trends on the periodic table:
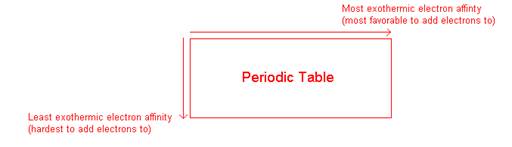
- How would you determine the electron affinity for Mg2+?
Look at the second ionization energy of magnesium.
Remember that electron affinity means adding an electron -
Mg 2+ + e - → Mg+
This reaction is the reverse of the second ionization energy of Mg.

So to determine the energy associated with the flip reaction – just change the sign of the ionization energy.
