The Ideal Gas Law Worksheet - Answer Key
Back to the other Gases Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- What is the Ideal Gas Law Equation?
PV = nRT
- From what laws is this equation derived?
- Boyle’s Law – relationship between pressure and volume
- Charles’ Law – relationship between volume and temperature
- Avogadro’s Law – relationship between moles and volume
- A sample of hydrogen gas has a volume of 8.56 L at a temperature of 0oC and a pressure of 1.5 atm.
- Calculate the moles of hydrogen present in the sample.
0.57 moles
- How would this answer change if the gas had been helium?
There would be no difference in the answer.
- What equation can you use if you are given a question in which there a change in state?
P1V1 = P2V2
n1T1 n2T2
- A sealed balloon is filled with 1.00 L of helium at 23oC and 1.00 atm. The balloon rises to a point in the atmosphere where the pressure is 220. torr and the temperature is -31oC. What is the change in the volume of the balloon as it ascends from
1.00 atm à 220. torr?
1.83 L
- A bicycle tire is filled with air to a pressure of 75 psi at a temperature of 19oC. Riding the bike on asphalt on a hot day increases the temperature of the tire to 58oC. The volume of the tire increases by 4.0%. What is the new pressure in the bicycle tire?
81.7 psi
- Assuming constant temperature and pressure what would the final volume be for the following reaction after it has run to completion?

19.3 mL
- What is STP?
An acronym that stands for Standard Temperature and Pressure.
Standard Temperature = 273K
Standard Pressure = 1 atm
- Consider

What mass of NaN3 must be reacted in order to inflate an airbag to 71.4 L at STP?
138g NaN3
- A compound has the empirical formula CHCl. A 256 mL flask, at 373K and 750 torr contains 0.800g of the gaseous compound. Give the molecular formula.
C2H2Cl2
- Consider:
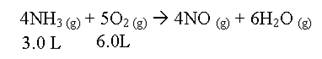
If this reaction is run at STP, what would the total volume be after this reaction has run to completion.
9.75 L
