Nomenclature Worksheet - Answer Key
Back to the other Coordination Compounds Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- Method for naming a coordination compound
- Cation is named before anion.
- Within the complex ion, name ligand before metal ion.
- Use prefixes to indicate the number of specific type of ligand attached.
- If the ligand is an anion, change the ending to “-o”.
If the ligand is neutral, use its name, as is.
- If more than one type of ligand, name them alphabetically.
- Indicate oxidation number of metal ion using (roman numerals) after metal name.
- If complex ion is anion, change ending of metal name to :-ate”.
- Fill in these naming charts
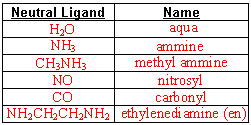
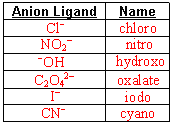
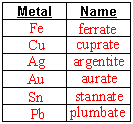
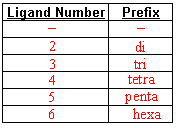
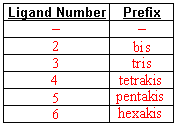
- Name the following
- [Co(NH3)6]Cl2
hexaamminecobalt (II) chloride
- K2[PtCl4]
potassium tetrachloroplatinate (II)
- [Co(H2O)6]I3
hexaaquacobalt (III) iodide
- [Co(NH3)3(NO2)3]
triamminetrinitrocobalt (III)
- Mn(NH2CH2CH2NH2)32+
trisethylenediaminemanganese (II) ion
- [Pt(NH3)4I2][PtI4]
tetraamminediiodoplatinum (IV) tetraiodoplatinate (II)
- Give the formulas for the following
- Sodium dicyanobis(oxalato)ferrate (III)
Na3[Fe (CN)2(C2O4)2]
- Tetracarbonyldihydroxochromium(III) ion
[Cr(CO)4(OH)2]+
- Triamminechloro(ethylenediammine)chromium (III) iodide
[Cr(NH3)3Cl(en)] I2
- Amminetrichloroplatinate (II) ion
[PtNH3(Cl)3]-
