Crystal Field Theory Worksheet - Answer Key
Back to the other Coordination Compounds Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- What is the crystal field model?
It is a model that views complex ions as being held together ionically
(this is not actually the case, but it allows for a simplification of the model).
The metal (M) is the cation and the ligands are negative point charges.
- Show the interaction between the d-orbital and the negative point charge ligands
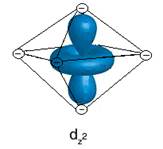
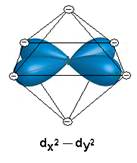
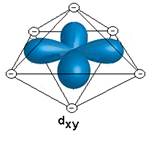

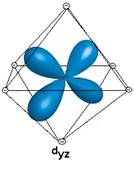
- Based on the diagrams above, would all of the d orbitals have the same energy? If not which would be higher energy?
No, dz2 and dx2 – y2 are higher in energy.
- The phenomena of d orbital splitting can happen two ways
- Large ∆E
- Small ∆E
- What affects the type of splitting that will happen?
● Charge on Metal
● Electronegativity of the Ligand (↑electronegativity, ↓∆E)
- General Ligand Ordering
CN- > NO2- > (en) > NH3 > H2O > -OH > F- > Cl-> Br- > I-
- The greater the charge on the metal the stronger the splitting.
- The Crystal Field Method can be used to explain the
- Magnetism
- Color (∆E)
- How does this model explain color?
Color that is seen is related to the ∆E between the separated d orbitals.
- How can we determine the color we will see?
Using the color wheel – we will see the color opposite to the one absorbed.
- What is the d-orbital splitting for
- Tetrahedral
- Square Planar

- Linear
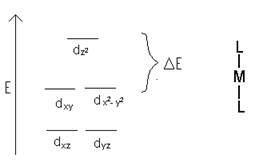
- How does the electronegativity of a ligand affect the d orbital splitting?
The more electronegativity, the lower the energy of the electrons, the more tightly bound the electrons are to the ligand.
- Compounds of copper(II) are generally colored, but compounds of copper(I) are not. Explain. Would you expect Cd(NH3)4Cl2 to be colored?
Cu+: 3d10 for this compound the d orbitals are full – meaning there is no room for the electrons to be promoted up. If electrons cannot be promoted up, there can be no release of energy when they fall back down – no release of energy means we see no color.
- Consider the complex ions Co(NH3)63+, Co(CN)63-, and CoF63-/ The wavelengths of absorbed electromagnetic radiation for these compounds are (in no specific order) 770 nm, 440 nm, and 290 nm. Match the complex ion to the wavelength of absorbed electromagnetic radiation.
CN– > NH3 > F–
(290 nm) (440 nm) (770 nm)
- How many unpaired electrons are in the following complex ions?
- Ru(NH3)62+ (low spin case)
There are no unpaired electrons.
- Ni(H2O)62+
There are 2 unpaired electrons.
- V(en)33+
There are 2 unpaired electrons.
- Draw the d-orbital splitting for the octahedral complex ions in each of the following.
- Fe2+ (high and low spin)
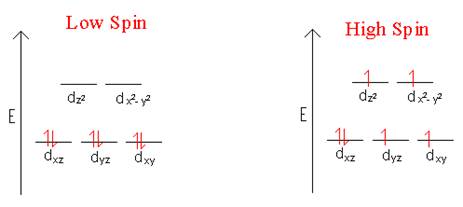
- Ni2+

- Zn2+
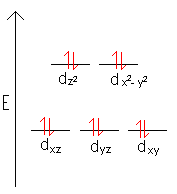
Weak field case always applies.

