Coordination Compounds Worksheet -
Answer Key
Back to the other Coordination Compounds Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- What is a coordination compound composed of?
- Metal Ion
- Ligand
- Counter Ion
- What is a complex ion?
The metal ion and ligand combination.
- What is a counter ion?
An ion that neutralizes the charge on the complex ion.
- What is a ligand?
A Lewis Base connected to a metal ion.
- What is a Lewis Base?
An electron donor. So the ligand must have a “free” lone pair.
- Two characteristics of many coordination compounds.
- Paramagnetic
- Colorful
- What is a primary valence? What does it correspond to?
Primary Valence = Oxidation Number
It corresponds to the charge on the metal ion.
- What is a secondary valence? What does it correspond to?
Secondary Valence = Coordination Number
It corresponds to the number of bonds on the metal.
- Define
- Monodentate
A ligand that attaches once (NH3, H2O, etc.)
- Bidentate
A ligand that attaches twice (H2NCH2CH2NH2)
- Polydentate
A ligand with multiple attachments.
- Chelate
A ligand with more than one atom that can bind to an ion. Capable of forming rings.
- A coordination compound of cobalt(III) contains four ammonia molecules, one sulfate ion, and one chloride ion. Addition of aqueous BaCl2 solution to an aqueous solution of the compound gives no precipitate. Addition of aqueous AgNO3 to an aqueous solution of the compound produces white precipitate. Propose a structure for this coordination compound.
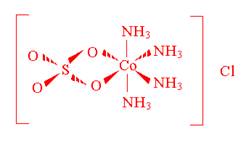
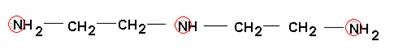
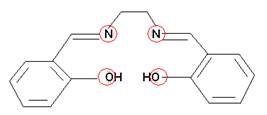
- How many bonds could each of the following chelates form with a metal ion?
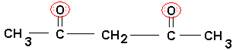
- Predicting the shape of a complex ion depends on the coordination number.
- When the coordination number is
- 2
- Linear
- 4
- Tetrahedral
- Square Planar
- 5
- Trigonal bipyramidal
- Square Pyramid
- 6
- Octahedral
