Ions Worksheet - Answer Key
Back to the other Basic Chemistry Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- What is an ion?
An atom or molecule that has an overall charge.
- How are ions formed?
An atom becomes an ion when electrons are lost or gained – thereby creating a disparity in positive and negative charge.
- What is a cation?
An ion with a positive charge.
- How is a cation formed?
A cation is formed by the loss of electrons.
- How is a cation named?
Using the element’s name from the periodic table.
- What is an anion?
An ion with a negative charge.
- How is an anion formed?
An anion is formed by adding electrons to an atom.
- How is an anion named?
An anion is named by replacing the suffix of the element name with “-ide” followed by the word ion.
- What elements on the period table tend not to form ions? Why?
Noble gases. They have a stable electron configuration as all orbitals are filled.
- What is ion most commonly formed by
- Alkali Metals: +1
- Alkaline Earth Metals: +2
- Group 3A : +3
- Group 5A : – 3
- Group 6A: – 2
- Halogens: – 1
- How is an ion indicated in a chemical symbol?
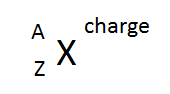
- Determine the number of protons, electrons and neutrons in the following.
- 56Fe3+
protons = 26
electrons = 23
neutrons = 30
- 19F-
protons = 9
electrons = 10
neutrons = 30
- 15N3-
protons = 7
electrons = 10
neutrons = 8
- 23Na+
protons = 11
electrons = 10
neutrons = 12
- In a neutral atom,
- The number of protons, electrons and neutrons are all equal.
- The number of protons and neutrons are equal. The electron count can be different.
- The number of neutrons and electrons are equal. The proton count can be different.
- The number of protons and electrons are equal. The neutron count can be different.
