Buffer Solutions Worksheet
Back to the other Acid Base Chemistry Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Answer Key - Solutions Manual
- What is a buffer solution?
- When solving problems for a buffer solution you can bypass the ICE chart and use the Henderson-Hasselbalch equation instead. What is the Henderson-Hasselbalch equation?
- Identify the buffer?
- HCl and NaClO4
- HClO and LiClO2
- HF and KF
- CH3NH2 and CH3NH3Cl
- Determine the pH of the following solutions
- 1.00 L solution of 1.00M HNO2 and 1.50M NaNO2.
(Ka = 4 x 10-4)
- 25.0g of NH3 and 40.0g NH4NO3 in 501.0 mL of water.
(Ka = 5.6 x 10-10)
- What is buffer capacity?
- Consider:
0.1 M NaF and 0.1M HF
1.0M NaF and 1.0M HF
0.01M NaF and 0.01M HF
- Which has the highest pH?
- Which has the highest buffer capacity?
- Consider a solution that contains both NH3 and NH4NO3. If a solution has a pH = 8.5, calculate the ratio of
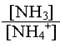
Ka = 5.6 x 10-10
- What volumes of 0.22M CH3COOH and 0.46M NaCH3COO must be mixed to prepare a 1.00L solution buffered at pH = 5.00? (Ka = 1.76 x 10-5)
