Liquids and Solids Practice Test - Answer Key
Back to the other Liquids and Solids Practice Tests and other General Chemistry Practice Tests
Go To -> Practice Test - Answer Key
- On Mount Everest, in Tibet, the atmospheric pressure is equal to 242 torr. At what temperature does water boil?
- 0oC
- 54oC
- 70oC
- 78.4oC
- 300oC
- What has a higher boiling point C2H5OH or CH3OH?
CH3OH
- Which of the following is the correct order of boiling points for NaNO3, CH3OH, C2H6, and Ne?
- Ne<CH3OH<C2H6<NaNO3
- NaNO3<CH3OH<C2H6>Ne
- Ne<C2H6<NaNO3<CH3OH
- Ne<C2H6<CH3OH<NaNO3
- C2H6<Ne<CH3OH<NaNO3
- Would the following molecules have the same or different viscosity? If different, who would have the higher viscosity?
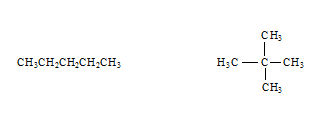
Different – CH3CH2CH2CH3 would be higher.
- An atom with mass 10-25 grams forms a cubic crystal with a density of 0.2 g/cm3 and a unit cell with a 10-24 cm3 volume. The crystal structure is:
- BCC
- FCC
- simple cubic
- none of the above
- The unit cell in a certain lattice of atoms X and Y consists of a cube formed by a Y at each corner of the cube, an X at the center of the cube and an X at the center of each face. The empirical formula of the compound is:
- X2Y3
- X5Y6
- X4Y
- XY
- Which of the following should have the highest boiling point?
- Na2O
- HF
- NH3
- N2
- H2O
- Which one of these liquids would you expect to have the highest vapor pressure at room temperature?
- water, H2O
- methanol, CH3OH
- ethanol, CH3CH2OH
- diethyl ether, CH3OH2–O–CH2CH3
- ethylene glycol, HO–CH2–CH2–OH
- Consider the following data concerning four different substances:
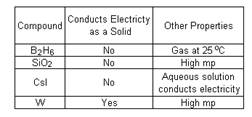
Label the four substances as either ionic, network metallic, or molecular solids.
B2H6 molecular solid
CsI Ionic
SiO2 network solid
W metallic
- The temperature inside a pressure cooker is 115oC. Calculate the vapor pressure of water inside the pressure cooker.
1.66 atm
- Mn crystallizes in the same type of cubic cell as Cu. Assuming that the radius of Mn is 5.6% larger than the radius of Cu and the density of Cu is 8.96 g/cm3, calculate the density of Mn.
d = 6.57 g/cm3
