Bond Theory Practice Test - Answer Key
Back to the other Bond Theory Practice Tests and other General Chemistry Practice Tests
Go To -> Practice Test - Answer Key
- Lewis Dot For:
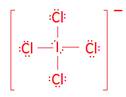
- ICl4-
- BeCl2
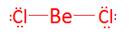
- HCN
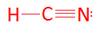
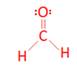
- H2CO
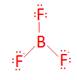
- BF3
- CO2
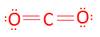
- Place the following in order of shortest to longest N-N bond length
N2 ,N2F4, N2F2
N2 < N2F2 < N2F4
- Determine the best Lewis structure for:
- OCN-
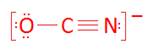
- NO
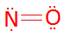
- What is the hybridization of the central atom in PCl4+?
- sp
- sp2
- sp3
- dsp3
- d2sp3
- What is the hybridization of the central atom in SO3?
- sp
- sp2
- sp3
- dsp3
- d2sp3
- For XeF2, correctly identify the electron arrangement around the central atom and the molecular shape.
- Octahedral and bent
- Octahedral and linear
- Trigonal bipyramidal and bent
- Trigonal bipyramidal and linear
- Tetrahedral and bent
- Which of the following species are paramagnetic?
- N2
- N2-
- N22-
- N2+
- N22+
- Order the following from the shortest to longest bond: C2, B2, H2, N2
- H2, N2, C2, B2
- N2, C2, B2, H2
- C2, N2, H2, B2
- C2, B2, H2, N2
- N2, H2, C2, B2
- For how many of the following does the bond order increase if one electron is removed from the neutral molecule?
B2, C2, P2 and F2 - 0
- 1
- 2a
- 3
- 4
- Which of these molecules has a measurable permanent dipole?
- H2
- CH4
- CO2
- NH3
- SF6
- Answer the following questions about PF given:
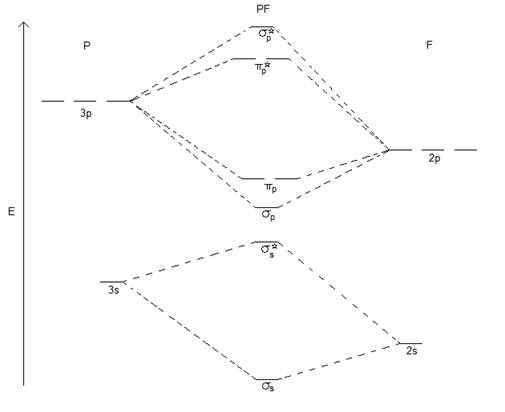
- What is the electron configuration for PF?
(σ2s)2(σ*2s)2(σ2p)2(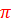 2p)4(π*2p)2
2p)4(π*2p)2
- What is the bond order of PF?
2
- Upon oxidation, PF+ can be formed. What is the bond order of this species?
2.5
- What is the magnetism of PF?
Paramagnetic
- If four orbitals on one atom overlap with four orbitals on a second atom, how many molecular orbitals will form?
- 1
- 2
- 4
- 8
- 16
- Which of the following statements is true?
- Electrons are never found in an antibonding MO.
- All antibonding MOs are higher in energy than the atomic orbitals of which they are composed.
- Antibonding MOs have electron density mainly outside the space between the two nuclei.
- Both statements a) and b) are true.
- Both statements b) and c) are true.
- Fulminate has the same formula as isocyanate (NCO-). However, these two ions have a difference in connectivity and drastically different properties, as one of them is, dangerously explosive. Predict which of these two ions exhibits this property.
- Fulminate (CNO-)
- Isocyanate (NCO-)
- How many sigma and pi bonds are in HCN?
σ: 2
π: 2
- Which of the following statements is false?
- Atoms or molecules with an odd number of electrons are always paramagnetic.
- Atoms or molecules with an even number of electrons are always diamagnetic.
- Paramagnetism cannot be determined solely from the Lewis structure of a molecule.
- Paramagnetic molecules are attracted towards a magnetic field.
- Two of the above statements are false.
- For IF4- determine
- Shape
Square Planar
- Polarity
Non-polar
- Hybridization of central atom.
d2sp3
- Dominant IMF
LDF
- How many unhybridized p orbitals and hybridized orbitals does an sp2 hybridized atom have?
3 hybridized sp2 orbitals and 1 unhybridized p orbital
- Which of the following statements is false?
- The molecule ClO2 can be accurately described by a Lewis structure consistent with the octet rule.
- The electrons in a polar bond are found closer to the more electronegative atom.
- A molecule with very polar bonds can still have no net dipole.
- Octahedral molecules never have a net dipole moment.
- More than one of the above is false.
- What is the hybridization of the central atom in IF5?
- sp
- sp3
- dsp3
- d2sp3
- Tetracyanoethylene has the skeleton shown here:
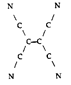
From its Lewis structure, determine the following.
- How many sigma bonds and how many pi bonds are in the molecule?
π: 9
σ: 9
- How many atoms are sp2 hybridized?
2 sp2 hybrids
- How many atoms are sp hybridized?
8 sp hybrids
- Which of the following has the shortest N-O bond?
- NO3–
- NO+
- N2
- NO2–
- none of these
- Which of the following statements is correct?
- A triple bond is composed of two σ bonds and one π bond.
- σ bonds result from the head-to-head overlap of atomic orbitals.
- Free rotation may occur about a double bond.
- σ bonds have electron density on the internuclear axis.
- More than one of these statements are correct
- The configuration (σ2s)2(σ2s*)2(π2py)1(π2px)1 is the molecular orbital description for the ground state of which of the following species?
- Li2+
- Be2
- B2
- B22–
- C2
- Which of the following species has the largest dissociation energy?
- O2
- O2–
- O22–
- O2+
- O22+
- Which of the following statements is true?
- Electrons are never found in an antibonding MO.
- All antibonding MOs are higher in energy than the atomic orbitals of which they are composed.
- Antibonding MOs have electron density mainly outside the space between the two nuclei.
- None of these statements is true.
- Two of these statements are true
- A compound, XF5, is 42.81% fluorine by mass. Identify the element X. What is the molecular structure of XF5?
X = iodine
square pyramid
- Consider three molecules: A, B and C. The central atom in molecule A has a hybridization of sp3. The central atom in molecule B has two more effective pairs than molecule A. The central atom in molecule C has two σ bonds and two π bonds. Give the molecular structure and hybridization around the central atom for each molecule.
A: sp3 tetrahedral
B: d2sp3 square planar
C: sp linear
